FDA announces advisory committee meeting on MDMA-assisted therapy
Read on for context about our recent petition to FDA; Lykos' response to ICER's report of "low confidence" in its research; and instructions for submitting testimony to the upcoming ad comm meeting.
Since I have a lot of new subscribers — some of whom clicked through from the recent Lykos editorial, as I’ll explain below — I’ll begin with a brief reminder and disclaimer. To be clear, I think that MDMA has potential as a therapeutic tool, and my life has been positively transformed by psychedelics. In the context of the upcoming FDA decision on MDMA-assisted therapy, my contention is that the MAPS/Lykos therapeutic adjunct increases the risks of MDMA in ways that have not been accurately reported or responsibly managed. I have been working to help educate the public about those risks, and to advocate for researchers to study those risks in a safer and more rigorous way.
Petitioning the FDA
In the aftermath of concerning signals from the psychedelics industry at SXSW (which was the focus of my last update), I was put in contact with a former MAPS PBC employee who identified problems with MAPS’s clinical trials of MDMA for post-traumatic stress disorder. (These trials form the basis for Lykos Therapeutics’ New Drug Application that is currently under review at the FDA, with an anticipated decision as soon as August.)
The former employee (FE1) described a number of issues with MAPS PBC’s clinical trials, which FE1 had been trying to communicate to FDA. One of the most concerning allegations involved systematic misreporting of adverse events, which included a serious suicide attempt during an MDMA dosing session.
As I had written in my SXSW recap, I was already concerned to hear Rick Doblin claim that the only instances of suicidal behavior only occurred in the placebo groups: “We had one woman [who] attempted suicide twice and another self-hospitalized with severe suicidal ideation, and both were in the placebo group. And so we didn't have any of this happen in the MDMA group.”
I had already spoken with multiple participants who attributed suicidal behavior and hospitalization to their experiences in MAPS’s MDMA dosing sessions, so I knew this characterization was inaccurate. More recently, I was sent a video of a participant discussing suicidal behavior after an MDMA dosing session (including a specific plan and actions taken toward that outcome) in front of MAPS researchers, including Rick Doblin. This suicidal behavior was not reported by MAPS.
Meanwhile, on another SXSW stage, Amy Emerson asserted that suicidality is just a “part of the process” of psychedelic therapy, but that the existing medical and regulatory systems need to be “taught” to embrace this.
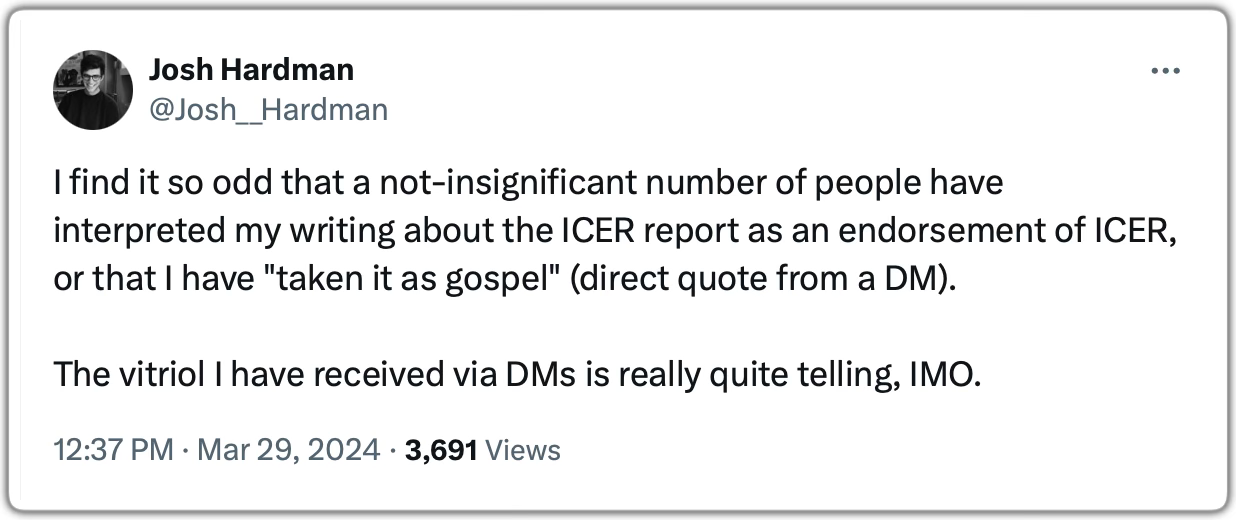
After I spoke with FE1, I sat with everything I’d heard and felt really heavy for a few days. I watched as the ICER draft report came out, which highlighted similar concerns with the research culture and its implications for adverse event reporting. And I saw how those who were publishing even neutral summaries of the ICER report were getting attacked online, as Josh Hardman of Psychedelic Alpha described in a tweet. In a by-now-familiar pattern, the industry was coming out in droves to minimize the concerns.
In the end, I decided that the new allegations were too serious to keep quiet. If the industry moved forward under these conditions and people ended up getting hurt, I would feel complicit if I didn’t say anything.
Several people who have seen problems at MAPS told me they can’t speak up publicly due to various vulnerabilities, including the threat of legal retaliation among those who have signed NDAs. In my case — for the first time in my life — I have a secure and stable job that is independent of the psychedelic medical industry. And because I could speak up, I felt that I had to. Thinking forward to the future, I wanted to know that I did what I could.
As a consequence, I organized the drafting of a petition to FDA, which was co-led by Meaghan Buisson with contributions from co-authors Alaina M. Jaster, Kayla Greenstien, and Brian Pace. (At the time of writing this, the petition has also been signed by 83 additional signatories.) Specifically, we asked the FDA to convene an advisory committee meeting with an extended open public hearing that allowed space for diverse stakeholders to share concerns about the safety and efficacy of MAPS/Lykos’ model of MDMA-assisted therapy.
Since it launched, the petition has been referenced in The Washington Post, NPR, Business Insider, DoubleBlind Magazine, and UC Berkeley’s The Microdose (2x).
From The Washington Post:
“Neşe Devenot, a psychedelics ethics researcher who co-authored the petition, cited published accounts of MDMA trial participants describing how their symptoms worsened afterward. ‘I just don’t think you can make a case that the data that they’ve provided to FDA is an accurate representation of what’s actually going on in the trials,’ Devenot, who has been critical of MAPS, said in an interview.” — David Ovalle and Daniel Gilbert in The Washington Post
From Business Insider:
“This April, a group of researchers including Devenot and Greenstien formally submitted a petition calling for the FDA to schedule an advisory meeting on Lykos' application because of concerns that the company had ‘normalized violations’ of industry safety regulations. The petition included an account from an anonymous former MAPS PBC employee about an incident where a participant made ‘a serious suicide attempt during a dosing session’ that was not reported. Two former MAPS employees who spoke with BI said they had heard about a participant attempting to run out into traffic during a session. They both confirmed this incident was not reported.” — Anna Silman in Business Insider
From NPR:
“The ICER report was followed in April by a citizen petition to the FDA. In that document, a group of concerned people allege possible misconduct and ethical violations that could compromise the MDMA research. The petition asked the agency to hold a public meeting to address the concerns. If true, the claims could jeopardize the drug's chances of receiving FDA approval, a decision that is expected to come by early August.” — Will Stone in NPR
Lykos responds to ICER
On April 29, Psychedelic Alpha published Lykos’ response to ICER as a guest editorial, signed by nine authors along with 63 additional signatories. In the introduction, the authors claim that my substack reveals evidence of an ideological agenda:
“One hundred and nine therapists and principal/co-investigators contributed to the Phase 3 trials of MDMA-AP for PTSD. To our knowledge, none of them were consulted before the preliminary report was issued. However, this group is in the strongest position to describe the studies and address accusations related to inappropriate study design and conduct. In the absence of such input, a number of assertions in the ICER report represent hearsay, and should be weighted accordingly. This consideration is particularly important because the two sources referenced in section 2.1, “Concerns About Trials of MDMA-AP” (pp 5-6) are a podcast and an online article written and produced by individuals who have repeatedly and publicly expressed strongly negative views about the medicalization of psychedelic substances, underscoring the high risk of bias in the preliminary report.”
Earlier this week, a journalist reached out to me with a question about the final claim, which directly links to my substack: “I saw in the MAPS clinician/investigator response to the ICER report that they cited you as someone ‘against medicalization of psychedelics’ and linked to this post. I couldn’t find any statements supporting that idea in your post.”
I responded to the journalist with the following: “You’re correct that the linked page does not support Lykos’ claims. In fact, during my presentation [at] SXSW, I mentioned that I encourage a multiplicity of approaches to studying potential uses of psychedelics, including medical applications. I specifically stated that I would have less concern about the psychedelic medical industry if the industry did not consistently participate in cover-ups of real risks, harms, dangerous therapeutic approaches, and patterns of abuse and assault. I link[ed] to that talk in the next substack entry.”
In a moment of irony that was noted by several readers of the Lykos editorial, most if not all of the signatories have professional conflicts relating to this research, since they are banking on the approval of MDMA-AT in order to establish careers in psychedelic therapy. The suggestion that an independent ethicist is more at “high risk of bias” demonstrates a lack of reflexivity about the authors’ own positionality.
I’m working in this area not because of some hidden agenda, but because I’ve directly encountered ends-justifies-the-means reasoning and behavior at the expense of vulnerable participants.
Furthermore, although hearsay is inadmissible in a court of law, this is not a court. If there are multiple and overlapping accounts of problems with the research, those problems should be investigated before the model is scaled. Lykos could cooperate with such an investigation by opening their data and by committing to release former employees from NDAs.
Although Lykos emphasizes that they were not consulted by ICER, the ICER report indicates that ICER had requested additional information from Lykos that was not provided, e.g.: “Data regarding the impact of MDMA-AP in terms of a change in the distribution of PTSD across stages of severity (i.e., asymptomatic, mild, moderate, severe) was requested from Lykos Therapeutics and not provided…. We requested both pre- and post-intervention EQ-5D scores from Lykos Therapeutics but did not receive these estimates” (p.32).
The editorial post on Psychedelic Alpha concludes by inviting readers to the virtual ICER meeting on 5/30, which will discuss their draft report. During the public portion of that meeting, I will be speaking about my concerns with the MAPS/Lykos therapy.
Obscuring the Inner Healer
Before the publication of the Lykos editorial, one of its authors had emailed me with stern words about our petition. (Hereafter, I will refer to this author as E.A.) They stated that while they were also concerned about the conduct of MAPS/Lykos, they were upset with my (mis-)characterization of the “inner healing intelligence,” which is MAPS’s proposed mechanism of action for MDMA-assisted therapy. (Notably, despite this major point of contention, the inner healer appears nowhere in the Lykos response to ICER.)
I’m excerpting from the exchange here, since it emphasizes how the Lykos editorial’s signatories are presenting a united front that does not accurately represent their discussions behind the scenes.
E.A. wrote that “I'm [not a] fan of Rick [Doblin] or MAPS/Lykos and want to know everything I can about the ways they have caused and continue to cause harm. And I totally agree with you that any problems with the MAPS research and data should be thoroughly exposed and discussed publicly before the FDA issues its ruling.” None of this concern was expressed in the Lykos editorial, which — to the contrary — presents an image of a tight academic consensus about the propriety and rigor of MAPS’s clinical trials. The editorial gives no indication that additional vetting of the data might be warranted.
E.A. offered to connect me with a group of MAPS clinical trial therapists “who have open eyes about the problems with MAPS/Lykos” and are “committed” to ensuring the ethical training of new clinicians once MDMA-AT is approved. The context suggested a widespread attitude that ‘we just need to get this across the finish line and can fix things post-approval.’ This would explain why many of these clinicians signed onto the Lykos editorial, which — to emphasize again — presented an appearance of rigorous consensus, with none of this recognized concern. (One named member of this group was also another author of the Lykos editorial.) This disconnect between the public image and MAPS/Lykos’ internal culture was more recently corroborated in Anna Silman’s investigative reporting for Business Insider.

Despite the acknowledged concern with MAPS/Lykos, E.A. emphasized that my assessment of the inner healer construct was damaging my professional reputation:
“I’m concerned about how you and your colleagues continue to attack ‘inner healing intelligence’…. From what I'm seeing and hearing, your public confusion about that issue is (1) seriously harming your credibility with competent, sophisticated and ethical clinicians and (2) will make it harder for you to achieve your stated goals…. [D]ismissive characterizations of inner healing intelligence as ‘unscientific’ — and as instead ‘spiritual’ and ‘religious,’ as if spiritual and religious principles are inherently unreasonable and disqualified from rational and intelligent discourse — are themselves based on a religious-type faith in positivistic and secular beliefs, values, and ideology, including the scientifically unprovable article of faith that only material, formal, and efficient causes are real and legitimate, but not final / teleological causes.”
I will excerpt directly from my response here, since it communicates my orientation towards these issues:
“My perspective is informed by years of careful thought and research on this subject.... My analysis doesn’t change based on social niceties or censures.... Although I believe you that your circles are not happy with the petition, other circles of thoughtful researchers have expressed unequivocal support for the petition.
“Disagreement with the petition is not an indictment of my scholarship. I chose to speak up on behalf of many others who felt they could not, because doing to aligns with my carefully considered values. I had accepted that some people would not like my professional opinions, and that had no influence on my decision to publish those opinions….
“You are welcome to write a separate statement where you narrowly support a hearing on allegations of research misconduct. This petition was written for others.”
It goes without saying that no alternative petition was written, and that the concerns of these MAPS trial therapists have not been publicly disclosed.
FDA solicits public comments on Lykos’ MDMA-AT application
After Lykos announced the advisory committee meeting in a May 6 press release, the FDA published its official “Notice of Meeting” on May 8. Importantly, this notice includes information about how members of the public can submit written comments to the advisory committee, which includes an option for confidential comments. It also includes instructions for requesting to provide an oral presentation during the open public hearing portion of the meeting, which will take place virtually “through an online meeting platform.”
Oral presentations: By Friday, 5/17, you must register your request to speak by contacting Joyce Frimpong. The request must include “a brief statement of the general nature of the evidence or arguments they wish to present, the names and addresses of proposed participants, and an indication of the approximate time requested to make their presentation.” Requests will be answered by 5/20.
Public written comments: By Thursday, 5/23, submit written comments to the Docket either electronically or by mail/delivered. Mailed/delivered comments must be received by 5/23. The full instructions are listed on the Notice.
Confidential written comments: By Thursday, 5/23, two paper copies of confidential comments must be received via mail/hand delivery/courier. Specific instructions for formatting these copies are listed on the Notice.
FDA asks me to withdraw our petition
The same day that the FDA published its “Notice of Meeting” for the advisory committee meeting, I received an email from an FDA lawyer asking if I would agree to withdraw the petition. An attorney who reviewed the FDA’s email identified it as highly unusual, since the FDA had not committed to honor the petition’s request for an extended open public hearing.
Re: Docket No. FDA-2024-P-2148
Dear Dr. Devenot:
We have received your citizen petition dated 4/28/24, requesting that FDA “Convene an Open Advisory Committee Meeting on MDMA-Assisted Therapy With an Extended Open Public Hearing to Include the Perspectives of Stakeholders Who Are Concerned About the Lykos Therapeutics New Drug Application’s Shortcomings and Risks”. Today, FDA announced that a public meeting of the Psychopharmacologic Drugs Advisory Committee will take place on June 4, 2024, from 8:30 a.m. to 4:30 p.m. Eastern Time. As explained in the notice, “The Committee will discuss new drug application 215455, for midomafetamine (MDMA) capsules, submitted by Lykos Therapeutics, for the proposed indication of treatment of post-traumatic stress disorder. The Committee will be asked to discuss the overall benefit-risk profile of MDMA, including the potential public health impact.” (see https://www.regulations.gov/document/FDA-2024-N-1938-0001)
Importantly, public input will not be limited to the one hour currently allotted for public comment. As the notice explains, members of the public may provide comments in the public docket FDA has opened for this advisory committee meeting, docket no. FDA-2024-N-1938; comments received by June 3, 2024, will be reviewed by the Agency. We will take under advisement your suggestion to expand the public comment time at the meeting, however, and the schedule may be adjusted prior to the meeting.
Under the circumstances, because the Agency has already announced the advisory committee meeting that was the subject of your petition, we request that you consider withdrawing your Petition. If you agree to withdraw the Petition, please let us know by Friday, May 17, 2024.
Best regards,
[Name Redacted]
In my response to the FDA, I emphasized that how the requests of our petition have not been met. Given the concerns we outline regarding safety and efficacy, an extended public hearing is especially important:
Dear [Name Redacted],
I do not consent for the petition to be withdrawn from the docket. As we mention in the petition, there is a high level of concern that Lykos has not accurately represented the safety and efficacy of its MDMA-assisted therapy in reporting to the FDA. As a result, it is important for the public record to reflect our requests, especially while our requests have not been met in full.
Our petition asks the FDA to extend the open public hearing to prioritize the inclusion of critical perspectives. It is impossible to have fulfilled this request while the advisory committee is still receiving applications for oral presentations, since we are requesting that invitations be broadly extended to those with evidence of issues related to safety and efficacy.
The public notice currently lists one hour for the open public hearing. According to the advisory committee regulations, the chair has discretion to allocate more than a single day to accommodate an extended open public hearing. We request that the advisory committee make use of this discretion to create parity between industry perspectives and concerned voices, including academic experts and those with relevant lived experiences.
Sincerely,
Neşe Devenot
Online petition updated with new form: “Record of Requests to Present Concerns”
The FDA’s “Notice of Meeting” indicates that it “may conduct a lottery” if the number of oral comment applicants exceeds the allotted time. This signals that the FDA might not prioritize “contributions from stakeholders who are concerned about the application’s shortcomings and risks,” as our petition requested.
As a consequence, we decided to update the online version of the petition with a new recordkeeping form. We established the form “to document requests to present concerns via oral comment,” which will serve “as a record of oral comment applicants to compare to the FDA's final list of speakers.” Please fill out this form if you intend to register interest in an oral comment to express concerns.
If you would like to add your name to the online petition, it’s still accepting new signatures.
Key takeaways
Specific harms are predictable with MAPS/Lykos’ MDMA-AT model, which means those harms are preventable. MAPS/Lykos hasn’t identified these risks, so I’m working with a team to explain these risks to ICER and to the FDA.
Ultimately, the psychedelic industry doesn’t actually depend on one company pushing through a dangerous therapy model via faulty research. And for me, “ends justify the means” doesn’t fly when people are getting hurt.
While they circle the [band]wagons, we’ll continue organizing. At a minimum, we will have a record showing that people tried to warn about this.
I’ll see you at the ICER meeting. -ND







Congrats Nese!
Really great work. After reading through the ICER comment report and seeing all of the experts in this field who are raising the same concerns it is obvious you are in good company.